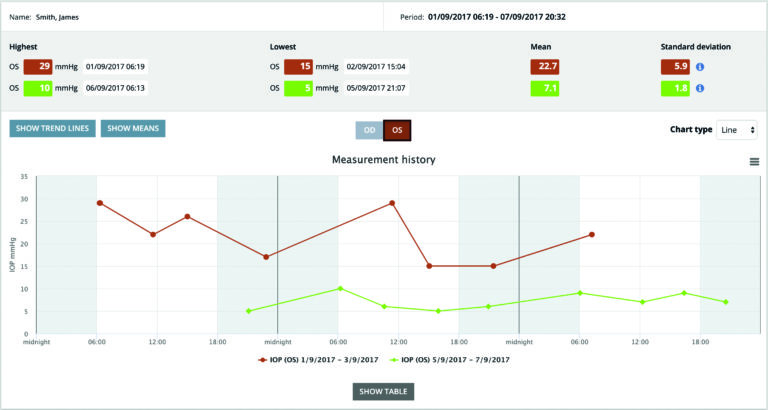
Storing and analysing tonometer data
iCare software solutions are evolving. Today a cloud-based iCare CLINIC is available for iCare HOME and iCare IC200 tonometers, for storing and analysing the patients’ IOP data. IOP measurement data can be uploaded easily to the cloud by using the iCare EXPORT and iCare PATIENT applications for PC and Android mobile devices.