iCare HOME tonometer for self-monitoring of IOP
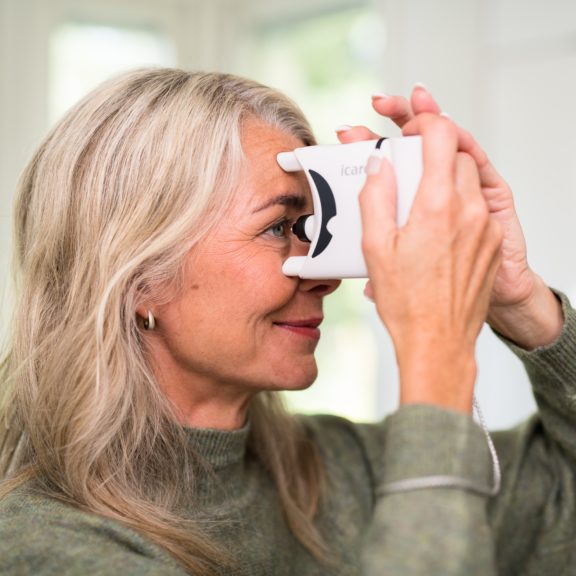
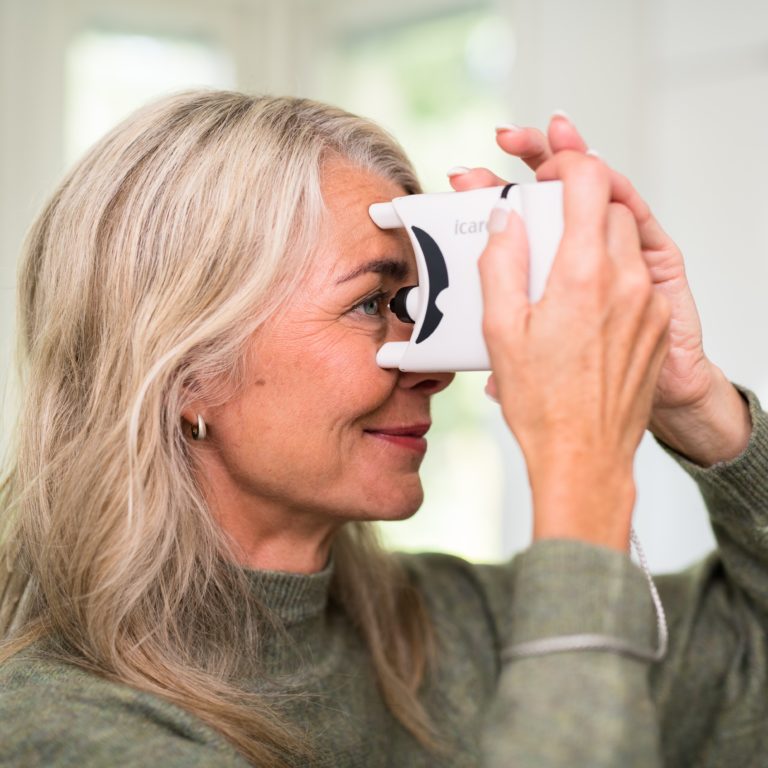
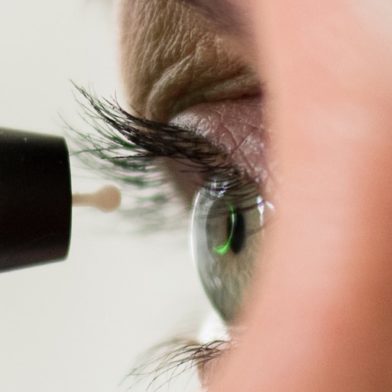
The iCare HOME tonometer is the first device available to patients for measuring intraocular pressure at home. iCare HOME provides an extensive and accurate view to the patient’s diurnal IOPs for the eye doctor adding value to glaucoma management. For the patient iCare HOME provides peace of mind and improves compliance to taking glaucoma medication.
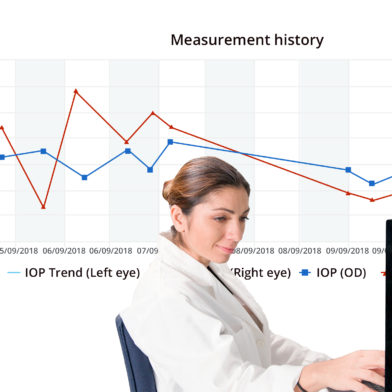
“The difference between readings we obtain in the office and the data from the Icare HOME is like the difference between a single snapshot and a continuous movie of a patient’s daily life.” – Iqbal Ike K. Ahmed, MD, FRCSC, Mapping diurnal IOP fluctuations at home, insert to Glaucoma Today, September/October 2018